Overview
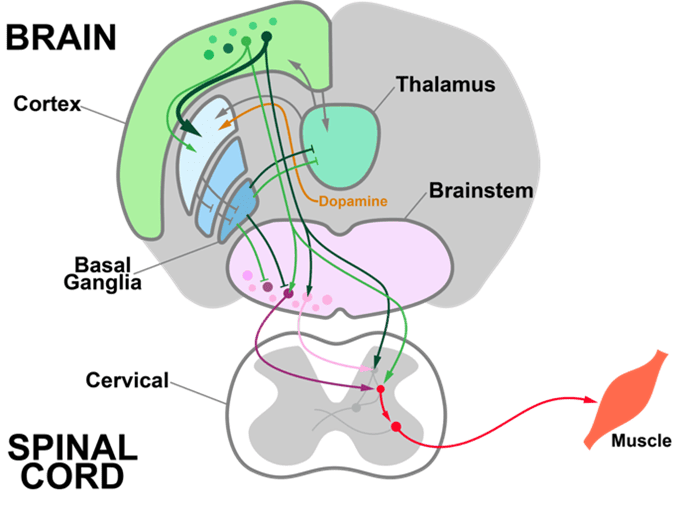
The human brain contains trillions of synapses. Through their patterns and strengths, these synapses are thought to encode our memories and acquired skills. How does the brain update its synapses to support behavioral learning without interfering with existing skills or memories? To study this question, we have developed i) optical connection-mapping techniques that leverage cellular-resolution two-photon (2P) optogenetics and calcium imaging in mouse motor cortex (MC) to track changes in the causal influence of each neuron in a recorded population; alongside ii) optical brain computer interface (BCI) learning tasks that explicitly define the causal relationship between imaged MC activity and behavior.
Optical Brain Computer Interface
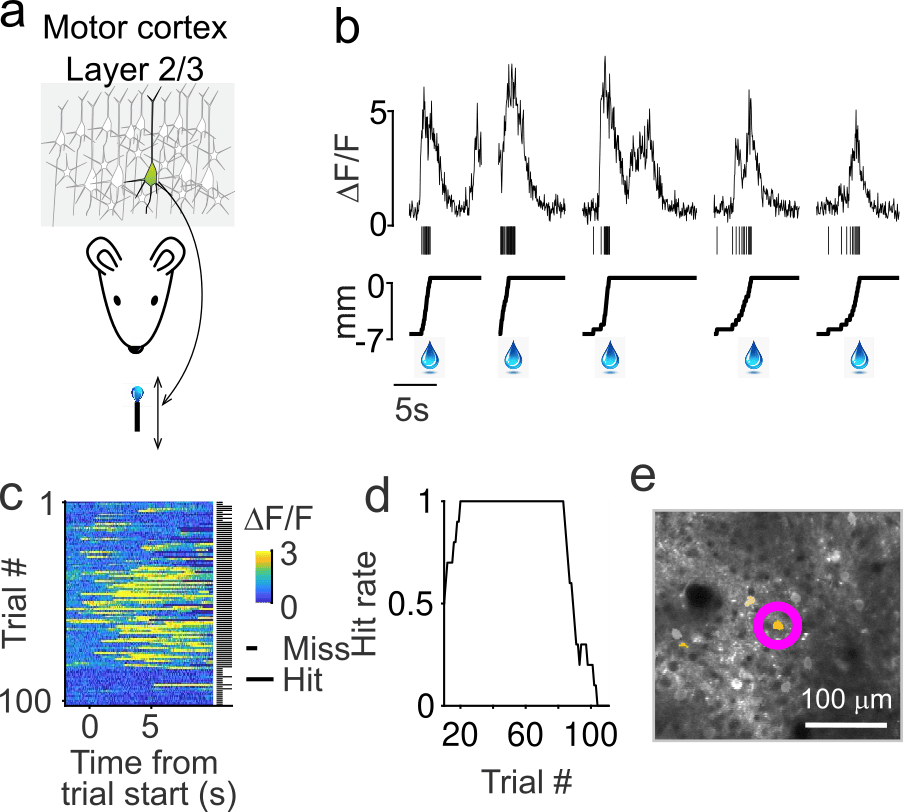
To simplify the study of learning, we developed an optical BCI task in which mice control the position of a motorized reward port with a single neuron in layer 2/3 of primary motor cortex (Figure 1). Mice quickly learn to increase the activity of this conditioned neuron (CN), resulting in increased reward rates in approximately 30 trials (5 minutes; Figure 2). Additionally, the changes in activity that follow learning are remarkably sparse: only a small fraction of neurons change their activity as much as the CN (Figure 3).
Learning rules
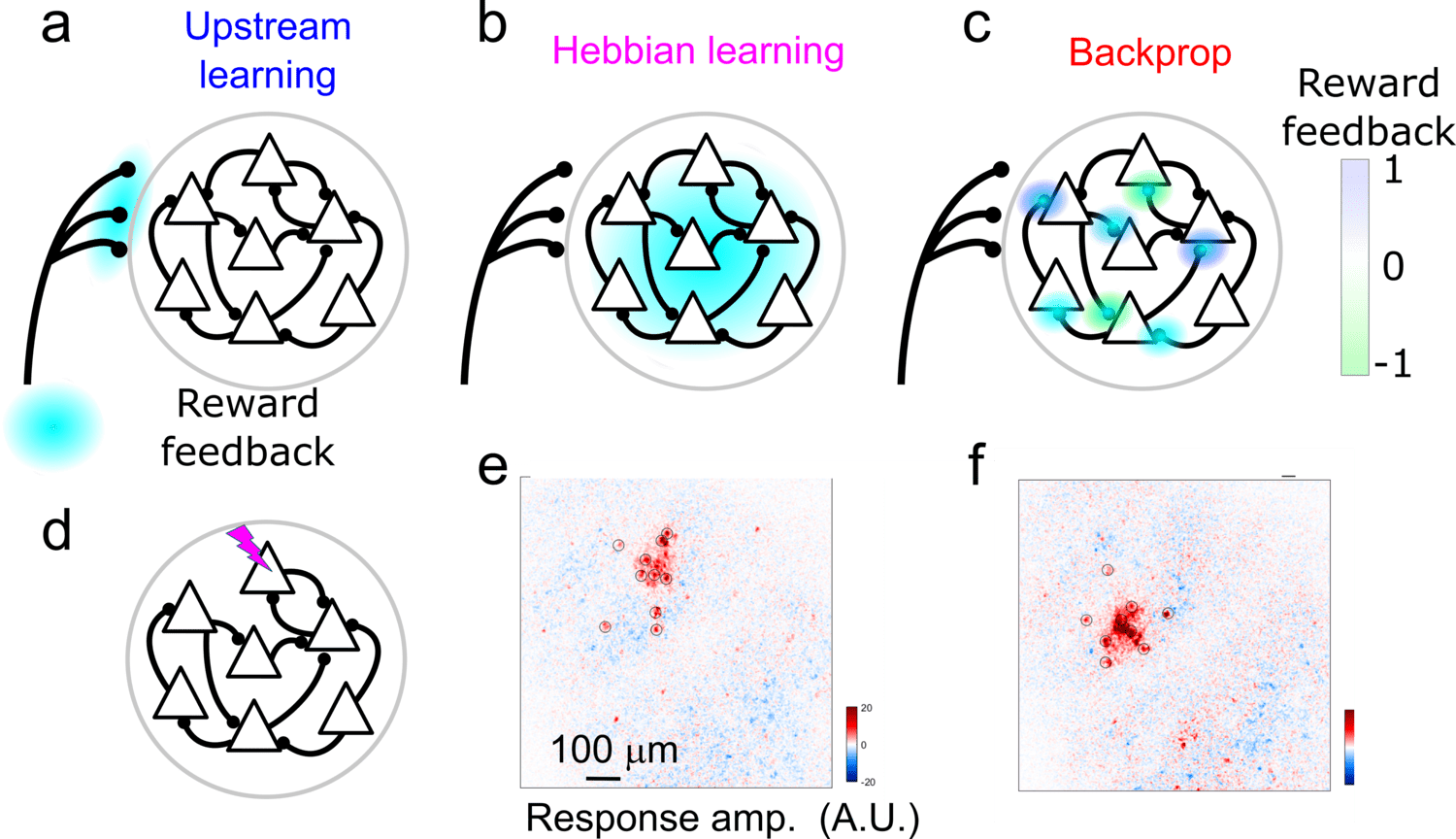
Activity changes in MC could result from either local circuit plasticity or plasticity in long-range inputs to MC. Our data demonstrate that BCI learning involves local circuit plasticity. What learning rules govern this plasticity? Two candidates are Error Backpropagation (Backprop), which leverages knowledge of the circuit's connectivity to optimally link inputs and outputs, and reward-modulated Hebbian-learning (3-factor learning), which finds input-output links through trial-and-error. To distinguish between these models, we use two-photon optogenetics to map causal connections between stimulated and non-stimulated neurons. Performing this all-optical “weight mapping” before and after BCI learning allows us to measure weight changes following learning.
Imaging neuromodulators during learning
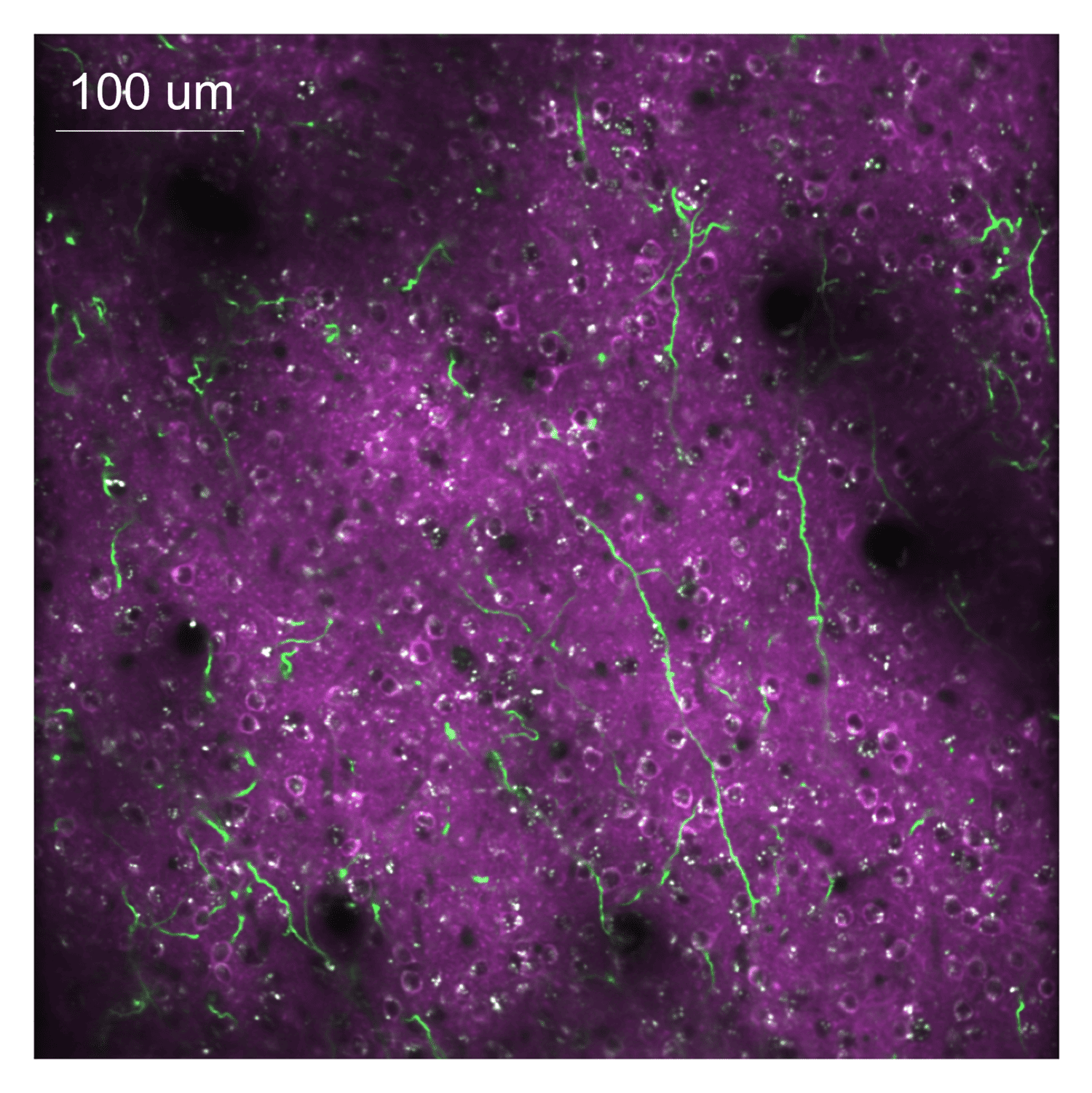
Weight mapping reveals that synaptic plasticity in motor cortex is modulated by an RPE-like signal. Recent work has suggested that norepinephrine may convey RPE information to cortical circuits. To determine if norepinephrine provides an RPE signal to the motor cortex, we perform functional imaging of NE axons originating from the Locus Coeruleus (LC) that express the genetically encoded calcium indicator GCaMP8s while mice learn the BCI task. In this experiment, activity in motor cortex is recorded using a red calcium indicator (jRGECO1a), which allows us to separate local somatic activity from LC axon activity (Figure 3). LC axons show activity that is strongly correlated with movements of the reward port, consistent with predictions from reinforcement learning models.
OpenScope Steering Committee
The OpenScope Steering Committee convenes at least biannually to provide crucial direction for the OpenScope project, playing an essential role in ensuring that we effectively serve our broader community.