Brain-Wide Anatomy at Cellular Resolution


Looking at information processing in brain-wide circuits requires a map of ‘connections’ between identified cell types that link brain regions. This includes long-range projection neurons and their classical synapses; neuromodulatory neurons and the neuron types that are within the ‘range of influence’ of their axons; and receptors and signaling complexes in these ‘postsynaptic’ neurons. The Multi-Scale Molecular Anatomy group (MSMA) uses innovative histology, imaging, and analysis techniques to map the connections and synaptic molecules of defined neuron types across brain regions.
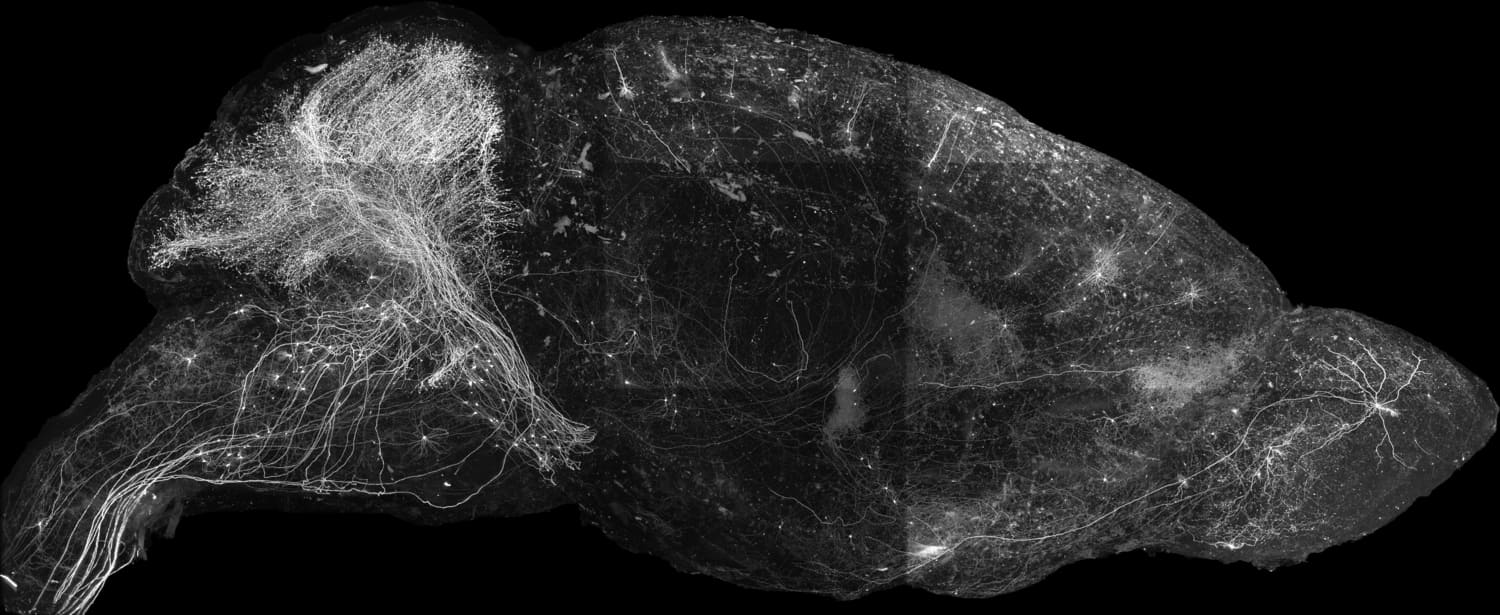
The mammalian brain is a complex 3-dimensional network of millions of interconnected neurons, and this intricate wiring underpins parcellations of the brain into functionally relevant brain areas. Individual neurons in these brain areas can be conceptualized as the nodes, and the synapses connecting them as the edges of these multi-regional circuits, orchestrating the complex directional flow of information that supports normal brain function and dysregulation in many neurological disease states. Computations performed by these multi-regional circuits therefore require looking at the long-range connections of individual neurons, their brain-wide collaterals, as well as the diversity of these neuronal ‘projection types’ within different brain areas. Such mapping of complete trajectories of mammalian neurons is a hard problem and poses some unique challenges—individual neurons traverse many millimeters to centimeters within the brain while individual axon collaterals can be finer than 100 nanometers, requiring microscopy methods that can image at nanoscale precision over centimeter spatial scales robustly without any loss of data. We are developing a next-generation technology stack for performing such high-resolution morphological reconstructions at scale and for analysis and classification of projection neurons in the mouse brain. This platform is based on the Expansion Assisted Selective Plane Illumination Microscope (ExA-SPIM) recently developed at the Allen Institute for Neural Dynamics that combines whole mouse brain or large specimen expansion followed by imaging on a custom large format light-sheet microscope that achieves resolutions needed for following fine axon branches in these very large specimens without the need for tissue slicing. We are developing variants of the expansion protocol that retain RNA and proteins over long time scales for post imaging interrogation of biomolecules of interest. Integral to our platform is a suite of automated data pipelines for data wrangling in the public cloud, machine learning for automated segmentation and accelerating reconstructions, a community portal for access to the reconstructed data, and tools for cloud-based data visualization, data sharing, and collaborative proofreading. We are using this platform to delineate the diversity of projection types in the non-sensory thalamus, sub-cortical inputs to the thalamus, and neuromodulatory systems.
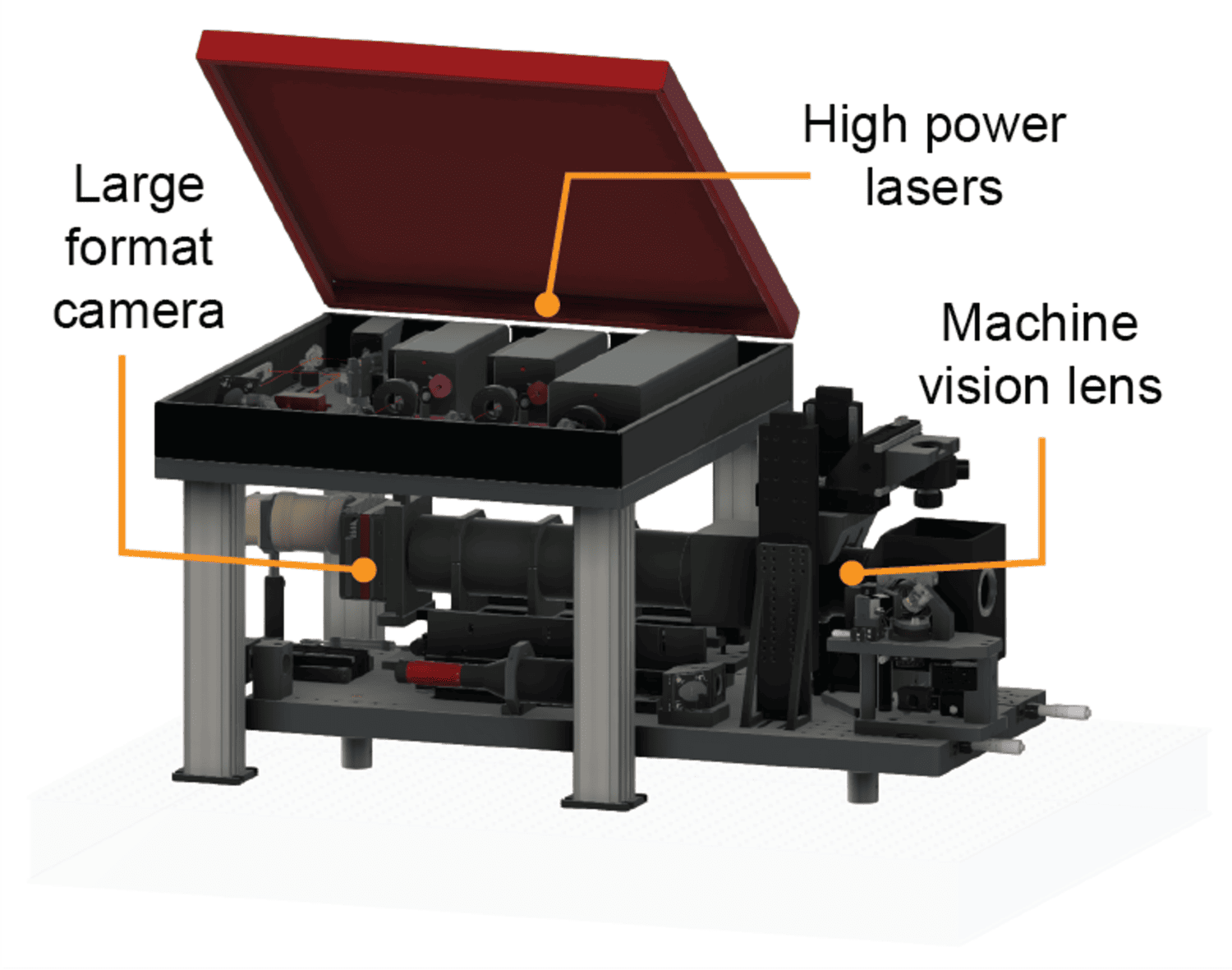
Tracing brain-wide projections of single neurons requires collecting whole mouse brain (WMB) imaging data at high -resolution and sufficient contrast, amounting to tens of teravoxels per brain without data loss. We use our ExA-SPIM single -neuron morphology platform to image and reconstruct complete morphologies of individual neurons.
This requires sparse and bright labeling of individual neurons with little fluorescence background. We use virally transduced fluorescent proteins in combination with a diverse set of transgenic driver lines and cell type- specific enhancer AAV tools that targets stochastic subsets of identifiable cell populations – —typically labeling a few hundred neurons per brain. We have developed methods for routine isotropic expansion of whole mouse brains rendering them spectacularly clear and suitable for imaging on the ExA-SPIM microscope. We have built a Brainwasher device that automates some of the routine liquid handling for whole brain clearing and labeling.
ExA-SPIM imaging achieves high-contrast, high-resolution WMB imaging at very high data rates. This microscope has a field of view ~100X larger (13.3 mm diameter) and a working distance ~10x larger (35 mm) compared to typically used systems for biological microscopy. A 3X expanded WMB can be imaged in only 15 tiles without sectioning at a near-isotropic effective optical resolution of 300 - 350 nm (lateral) and 800 - 850 nm (axial), and the microscope can image an expanded brain ~50 x 25 x 18 mm3 in less than one day. The detailed parts list and designs of the microscope are open source. To complement the hardware and support the large-scale and high-speed imaging, we have developed custom acquisition software ‘‘Acquire’ in collaboration with CZI that interfaces directly with camera drivers and streams imaging data at high rates and over long durations directly to next-generation file formats (OME-Zarr V2/V3).

The scale (100 TB / brain / channel) and throughput (up to 1.8 GB/s) of the ExA-SPIM requires efficient data management. To facilitate imaging and processing multiple samples per week (500 TB / week), we require both software and hardware that scales rapidly and flexibly. We have built automated data pipelines for lossless compression, metadata capture, and moving data efficiently from an on-premises enterprise storage server (VAST Data) to open AWS S3 buckets. In addition, we have built pipelines for cloud-based data processing, including stitching, fusing, and quality control.
Manual reconstruction of neuronal arbors is a slow process, exacerbated by the presence of multiple labeled neurons: the dendritic and axonal branches from different neurons can course close to each other, sometimes within the resolution of the microscope. Tracing neurites thus demands careful inspection of very large image volumes. Scaling up the overall pipeline requires automation of neuron reconstruction. In collaboration with Google Applied Sciences, we have implemented a flood filing networks (FFN)- based segmentation pipeline followed by a graph neural network (GNN)-based postprocessing correction of segmentation errors. To train and validate our machine learning models, we have collected expert-annotated ground-truth data.
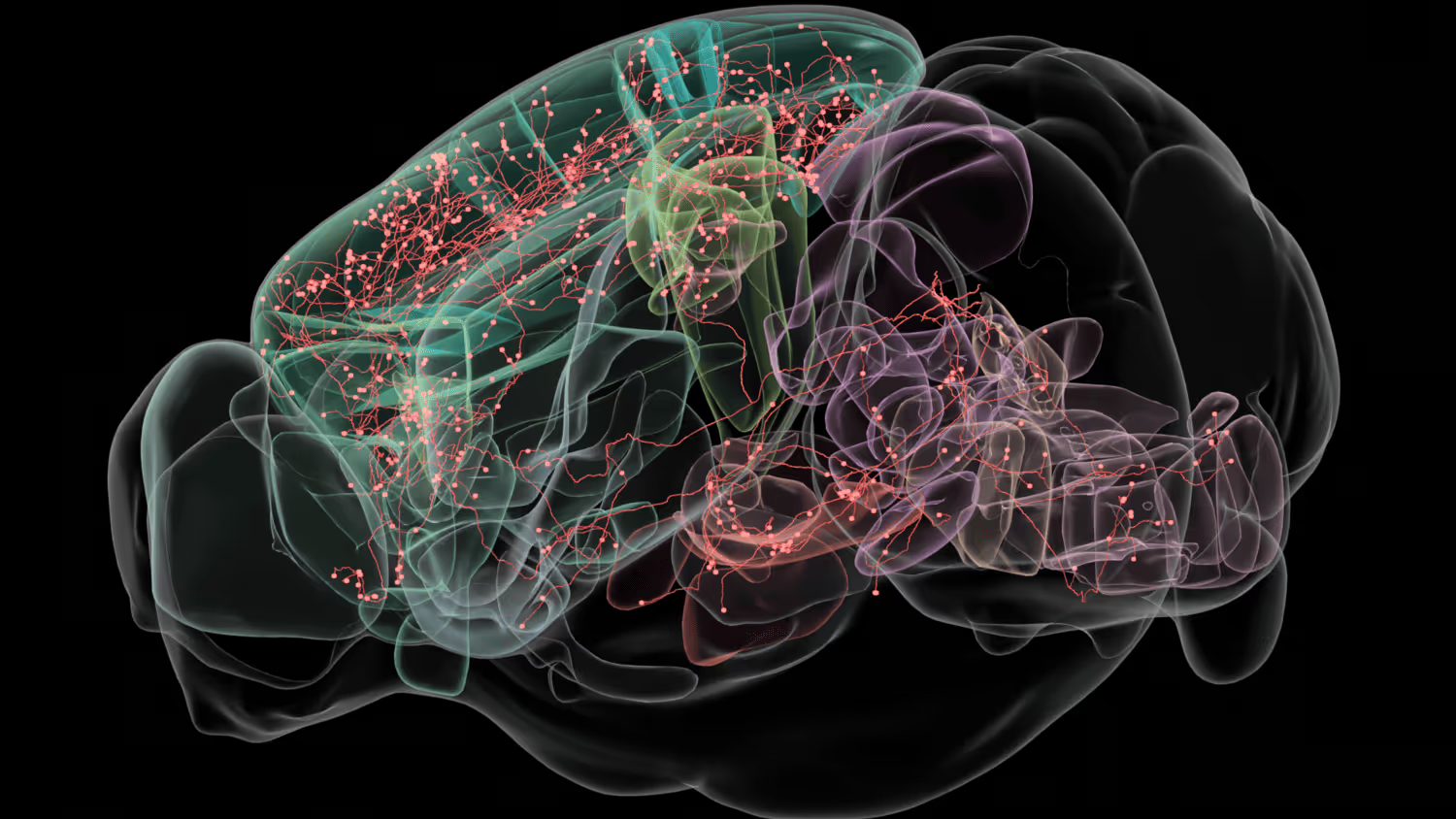
Detailed characterization of defined circuits requires domain-specific expertise. We have established two cloud-based pipelines for visualization and interactive analysis of WMB data stored in our public AWS S3 buckets. We visualize image data using Neuroglancer, a cloud-native, high-performance tool for large-scale, multi-resolution 3D images. Sharing data with collaborators simply involves sharing a URL. Efficient and ergonomic proofreading requires software with good 3D image rendering, performance, and support for complex annotation workflows. In collaboration with the Janelia Research Campus team, we have extended the capabilities of HortaCloud software to natively support cloud-friendly data formats such as OME-Zarr and added tools for semi-automated proofreading. This allows open data analysis from any geographic location, without the need for individual groups to create resource-intensive datasets themselves, invest in purchasing expensive desktop computers with high-end GPU cards, or move large datasets between locations.
We store all data in the standards-compliant OME-NGFF file format and in public S3 buckets, making sharing and data visualization straightforward. We are currently building a searchable web portal that will enable users to identify neurons of interest, view and work with fully reconstructed neurons, or link to primary WMB image data in Neuroglancer, ITK widgets, and other browser-based visualization applications.
The Surgery team offers a variety of aseptic rodent surgical procedures ranging from stereotaxic injections to headpost implantation and cranial windowing.
The Neuropixels platform uses pioneering technology for highly reproducible, targeted, brain-wide, cell-type-specific electrophysiology to record neural activity from defined neuron types across the brain.
The Molecular Anatomy platform combines innovative histology, imaging, and analysis techniques to map the morphology and molecular identity of neuron types across the whole brain.
The Fiber Photometry platform enables optical measurement of neural activity in live animals to study neural circuits' function and dynamics in behaving animals.
The Behavior platform uses advanced technology to implement a standardized, modular, multi-task virtual reality gymnasium for mice, with the goal to study brain function across different behaviors at scale.