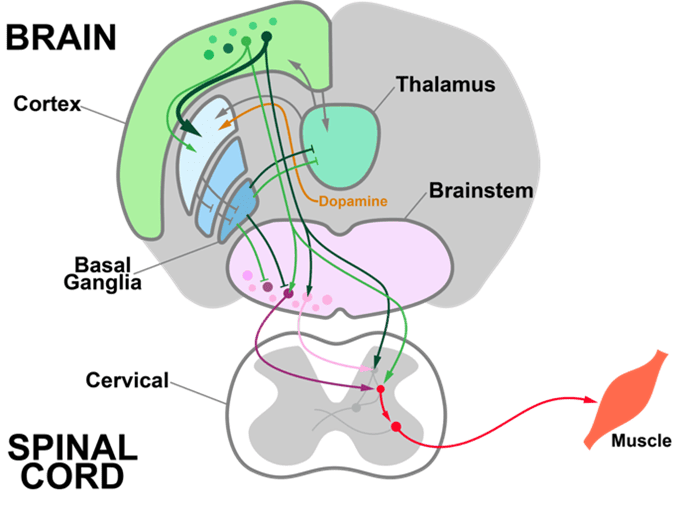
Neurons display remarkable diversity across multiple dimensions, including morphology, connectivity, gene expression, and biophysical properties; yet the relationship between cell type diversity and the various functions of the brain remains mysterious. Learning is one of the brain’s most essential functions, crucial for adapting to a constantly changing environment and optimizing behavior. How does the activity of transcriptomically defined cell types evolve during task learning? How are interactions among cell types restructured during novel experiences, or with increasing levels of task difficulty? Understanding these dynamics will provide key insights into how neural circuits are organized and how their coordination contributes to brain function.
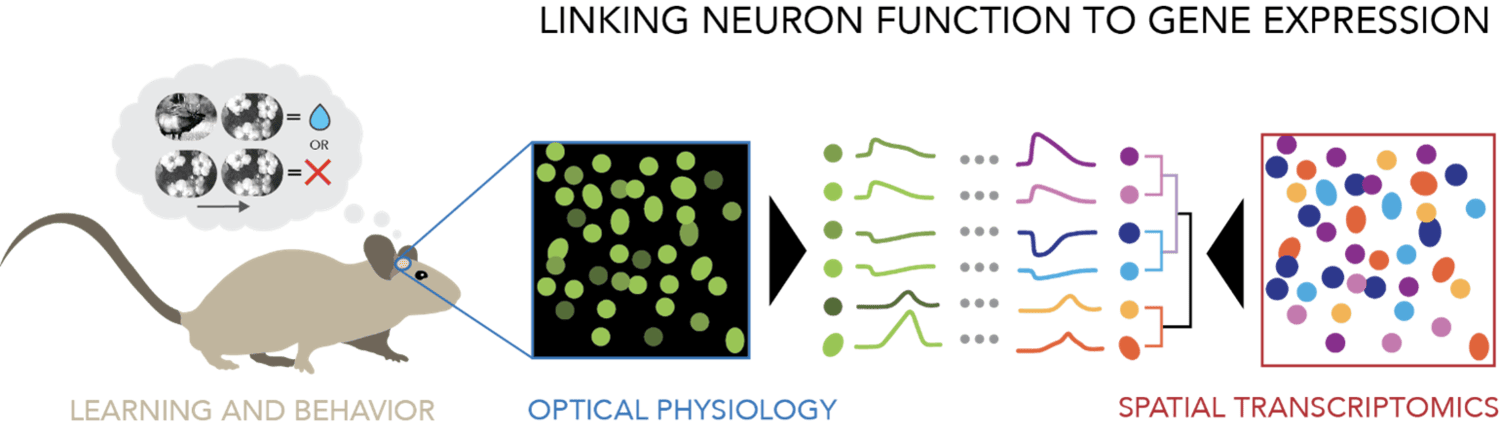
The Cell Types & Learning project aims to study the dynamics of cell type-specific activity during task learning, using longitudinal calcium imaging combined with post hoc spatial transcriptomics. Our goal is to understand how neural coding and circuit interactions evolve over time as mice learn to perform a visually guided task and to connect these physiological properties with the diversity of brain cell types.
Longitudinal Imaging during Learning
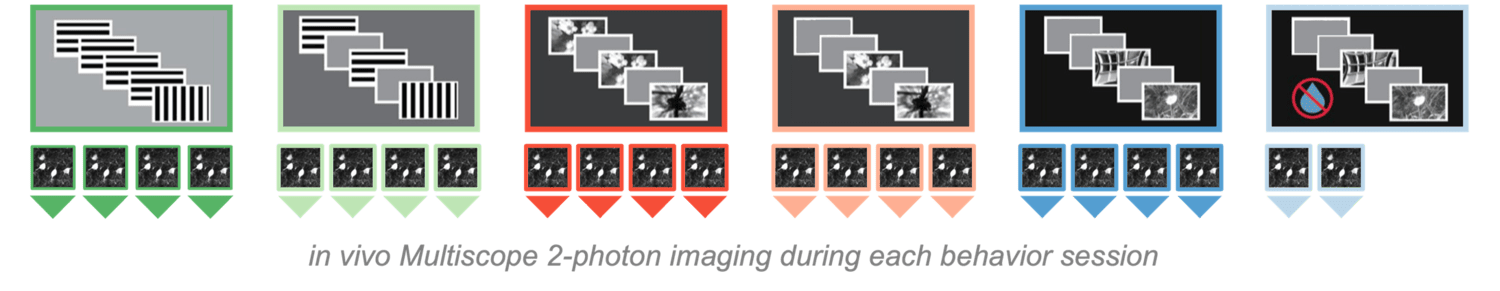
To link changes in behavior with changes in neural coding and dynamics, we use multi-plane, 2-photon calcium imaging to record the activity of hundreds of individual neurons over weeks during task learning. During imaging, we train mice to perform a visual change detection task, where they learn to attend to a stream of repeated sensory information and report when the stimulus identity changes. The task is learned over multiple training stages that incorporate increasing levels of complexity and different forms of learning. These include generalization of behavior to novel stimuli, relying on working memory after a delay, and extinction of learned behavior when stimulus and reward are dissociated.
In addition to functional recordings, we acquire high-resolution three-dimensional structural images to facilitate the alignment of in vivo physiology to ex vivo tissue sections used for spatial transcriptomics.
Spatial Transcriptomics
To understand how specific cell types contribute to shifting activity dynamics during learning and novelty processing, we use spatial transcriptomics to map gene expression of the neurons that were recorded in vivo to determine their molecular identity. This relies on the ability to find and visualize the same neurons across both imaging modalities. To solve this technically challenging problem, we use a method that involves imaging optically cleared and expanded thick tissue sections, which has the benefit of preserving cell morphology and position to facilitate 3D registration to structural images acquired in the intact brain.
.png)
Measurement of gene expression is achieved by labeling individual mRNA transcripts with fluorophores through HCR (Hybridization Chain Reaction) and visualized by lightsheet imaging. Over successive rounds of HCR and imaging, we can quantify the expression profiles of dozens of genes, which are used to classify the identity of each cell in reference to well-established cell-type taxonomies.
OpenScope Steering Committee
The OpenScope Steering Committee convenes at least biannually to provide crucial direction for the OpenScope project, playing an essential role in ensuring that we effectively serve our broader community.